Voltage-gated NaV channels (NaVs) initiate action potentials in excitable cells and are crucial for electrical signaling from bacteria to man. Voltage-gated CaV channels (CaVs) are activated by depolarization during action potentials, and Ca2+ entry through them initiates synaptic transmission, muscle contraction, hormone secretion, and many other biochemical and physiological processes. These channels share similar voltage-dependent activation and inactivation processes, whose structural basis is fundamental for electrical signaling.
Sodium channels are integral membrane proteins that form ion channels, conducting Na+ through a cell’s plasma membrane. They are classified according to the trigger that opens the channel for such ions, i.e. either a voltage-change or a binding of a substance to the channel. In excitable cells such as neurons, myocytes, and certain types of glia, sodium channels are responsible for the rising phase of action potentials. Voltage-gated Na+ channels can exist in any of three distinct states: deactivated (closed), activated (open), or inactivated (closed). Ligand-gated sodium channels are activated by binding of a ligand instead of a change in membrane potential.
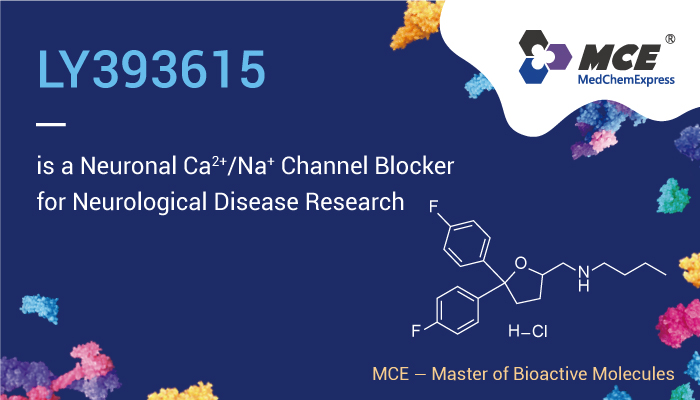
LY393615 is a neuronal Ca2+ and Na+ channel blocker
LY393615, a novel neuronal Ca2+ and Na+ channel blocker with neuroprotective effects in models of in vitro and in vivo cerebral ischemia. Therefore, LY393615 inhibits calcium flux in HEK 293 cells with α1A and α1B calcium channel subunits. And it inhibits P-type calcium channels in isolated Purkinje cells. LY393615 protects against hypoxia-hypoglycaemic insults in brain slices and also provides significant protection against ischaemia-induced hippocampal damage in gerbil global cerebral ischaemia. Meanwhile, LY393615 also produces a significant reduction in the infarct volume following Endothelin-1 (HY-P0202) middle cerebral artery occlusion in the rat. What’s more, LY393615 has good blood-brain barrier permeability.
In conclusion, in the present studies we have reported that a novel calcium channel blocker, LY393615, with good bioavailability protects against neuronal damage caused by hypoxia-hypoglycaemia in vitro and both global and focal cerebral ischaemia in vivo. The compound is neuroprotective when administered post-occlusion and may therefore be a useful anti-ischaemic agent.
References:
[1] Catterall WA, et, al. Trends Biochem Sci. 2015 Sep;40(9):526-34.
[2] O’Neill MJ, et, al. Brain Res. 2001 Jan 5;888(1):138-149