Immune-mediated necrotising myopathies (IMNM) belongs to idiopathic inflammatory myopathies (IIMs) which encompass a group of acquired muscle disorders. Including dermatomyositis, inclusion body myositis, polymyositis and overlap myositis. However, IMNM are rare and severe diseases with symmetrical and proximal muscle weakness, elevated levels of creatine kinase reflecting the degree of muscle cytolysis and myogenic patterns in electromyography. According to a large amount of research data, IMNM is associated with pathogenic anti-signal recognition particle (SRP) or HMGCR antibodies, at least partly through activation of the classical pathway of the complement. Hence, we will introduce Zilucoplan (RA101495). Zilucoplan is a component 5 (C5) Inhibitor for IMNM research.
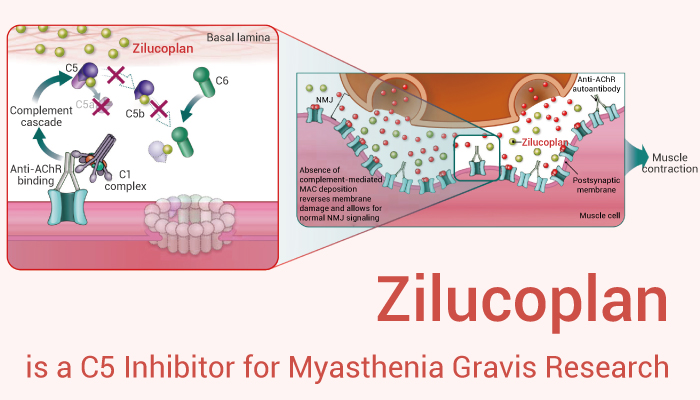
Zilucoplan is a C5 Inhibitor for Myasthenia Gravis Research.
In vitro, Zilucoplan (1-1000 nM; 30 min) inhibits Lipopolysaccharides-induced increase in C5a plasma levels in human whole blood (IC50=474.5 pM). Moreover, Zilucoplan has a 65.7% reduction in C5a plasma levels observed at a concentration of 1 nM. Besides, Zilucoplan bins to complement C5 and blocks the downstream assembly of the membrane attack complex (MAC; C5b-9) by inhibiting the cleavage of C5 by the C5 convertase into C5a and C5b and binding to preformed C5b to sterically block interaction with C6, thereby inhibiting the formation of membrane pores and subsequent cell death.
Zilucoplan also shows good activities in vivo. As research artical demonstrated that Zilucoplan (10 mg/kg; S.C.; daily, for 6 d) prevents the development of IMNM in C5-deficient mice supplemented with human complement. In addition, Zilucoplan (10 mg/kg; S.C.; daily, for 6 d) has protection on myopathy prevention in C57BL/6 mice.
In a word, Zilucoplan is a promising C5 inhibitor for IMNM research.
Reference:
[1] Julien S, et al. Biomedicines. 2022 Aug 20;10(8):2036.
[2] Gorman DM, et al. Amino Acids. 2021 Jan;53(1):143-147.