Sleep is indispensable for most animals. Humans spend nearly a third of their lives asleep, yet the exact functions and mechanisms of sleep remain contested. Sleep plays a crucial role in many biological processes, including immune regulation, cognitive performance, and metabolism.
Insufficient sleep can cause numerous health issues. However, the specifics of how sleep regulates immunity and how sleep deprivation leads to health problems are yet to be elucidated.
This article presents a revised sleep deprivation (SD) model for mice termed “curling prevention by water” (CPW). It mirrors the previously described disk-over-water (DOW) model used in rats. The CPW model significantly reduces both rapid eye movement sleep (REMS) and non-rapid eye movement sleep (NREMS) in mice over an extended period (>4 days). This prolonged SD triggers serious systemic inflammation and premature death in mice.
Prolonged sleep deprivation induces a cytokine-storm-like syndrome in mammals
The scientists examined the role of cytokines in sleep deprivation (SD)-induced death. Researchers administered an anti-cytokine-storm agent such as acetaminophen (APAP) or corticosterone (the primary glucocorticoid for rodents) to mice undergoing sleep deprivation. This treatment took the form of additions to their drinking water. Moreover, they orally supplied MK-571 sodium (0.1 mg/mL in ddH2O) via the same drinking water to these treated groups.
Futhermore, lipoic acid is an antioxidant that neutralizes ROS via electron donation36 and protects against lethality in sleep-loss flies. Treating these mice with lipoic acid failed to protect against the mortality of mice exposed to the CPW paradigm compared with vehicle controls. ABC transporters located on the luminal side of capillary epithelial cells mediate active efflux across the BBB. These transporters generally include ABCA2, ABCB1, ABCCs, and ABCG2.
Researcher administered two ABC antagonists-tariquidar, which targets ABCB1, and MK-571, which targets ABCCs-to mice during SD. Only MK-571 protected against SD-induced mortality. It suggests SD enhances the efflux of ABCC substrates, which subsequently trigger systemic inflammation.
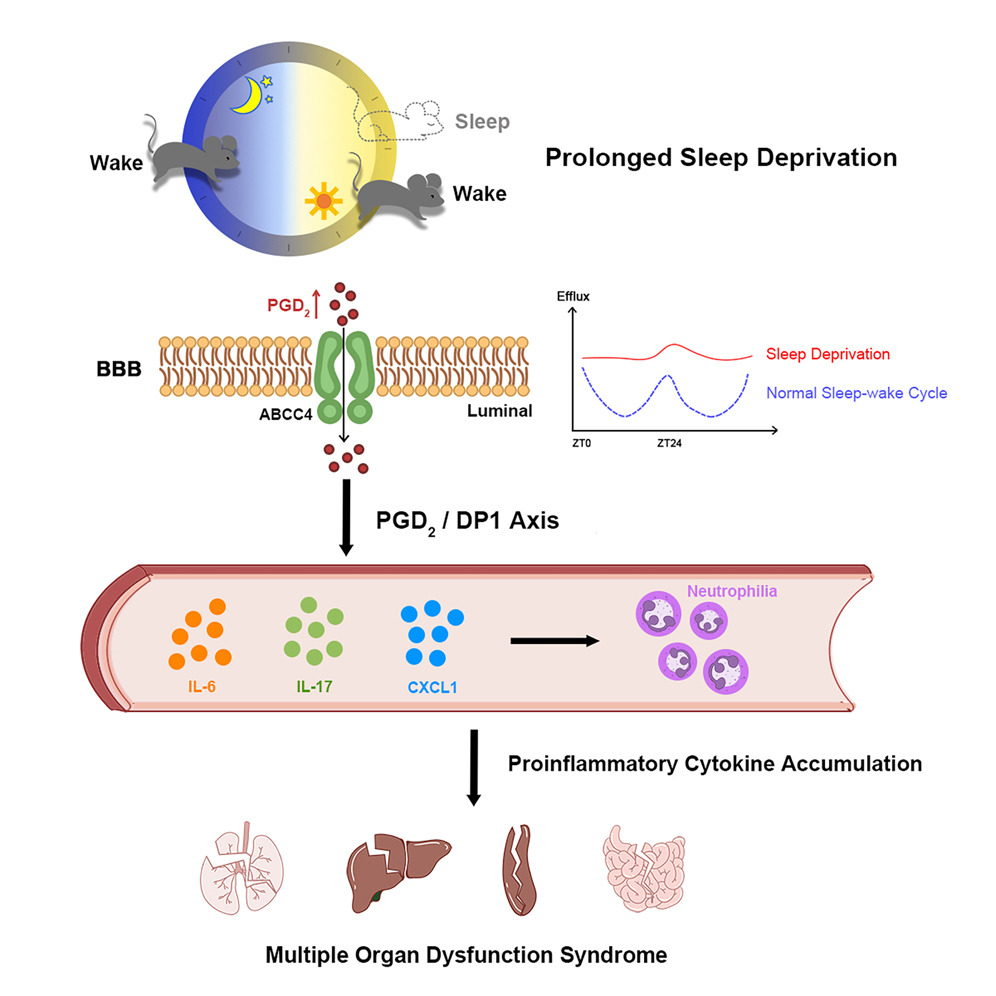
Summarily, systemic inflammation observed during sleep deprivation resembled a cytokine-storm-like state, accompanied by multiple organ dysfunction syndrome (MODS), increased expression of various proinflammatory cytokines, and build-up of circulating neutrophils. The cause of these immune responses is the accelerated outflow of prostaglandin D2 (PGD2) across the brain-blood barrier via the ATP-binding cassette subfamily C4 transporter (ABCC4). Blocking this PGD2/ PGD2 receptor 1 (DP1) axis, either pharmacologically or genetically, dramatically reduced SD-induced inflammation.
Reference:
[1] Sang D, et al. Cell. 2023 Dec 7;186(25):5500-5516.e21.