On November 15, 2023, the U.S. Food and Drug Administration has approved Defencath (taurolidine and heparin) catheter lock solution (CLS). CLS can reduce the incidence of catheter-related bloodstream infections in adults with kidney failure receiving chronic hemodialysis through a central venous catheter.
Renal failure results in inability of the kidneys to excrete waste products and extra water from the body. To replace the kidney function, patients with renal failure must adopt a treatment option which includes hemodialysis, peritoneal dialysis or kidney transplant. Hemodialysis is a method to filter wastes and extra water from the blood. However, patients undergoing hemodialysis often use a catheter for the filtration process which can lead to risk of bloodstream infection. Therefore, reducing the risk of microbial infection through a patient’s catheter can reduce the risk of bloodstream infections.
Defencath (taurolidine and heparin) CLS is available as a sterile, preservative-free, clear, aqueous-based solution.
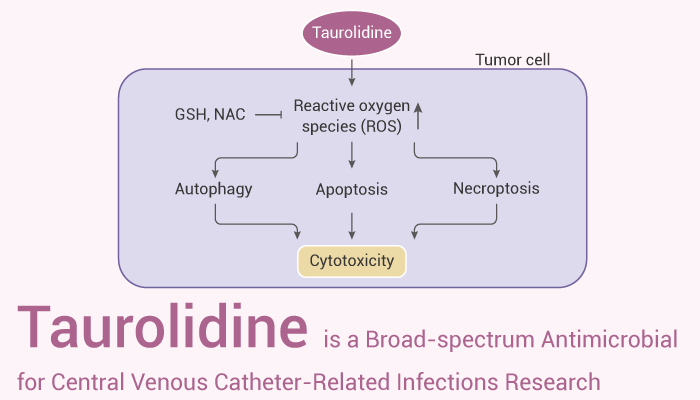
Taurolidine is a broad-spectrum antimicrobial for the prevention of central venous catheter-related infections. In addition, taurolidine has a direct and selective antineoplastic effect on brain tumor cells by the induction of apoptosis. Heparin is a highly sulfated glycosaminoglycan, that is widely used as an injectable anticoagulant.
Defencath was studied in a single, randomized, active-controlled phase 3 clinical trial. In this trial, Defencath delayed the time it took to acquire a catheter related bloodstream infection (CRBSI). Defencath demonstrated a 71% risk reduction in CRBSIs versus the heparin comparator arm.
Reference:
[1] Haro C, et al. Rev Chilena Infectol. 2019 Aug;36(4):414-420.
[2] Capila I, et al. Angew Chem Int Ed Engl. 2002 Feb 1;41(3):391-412.