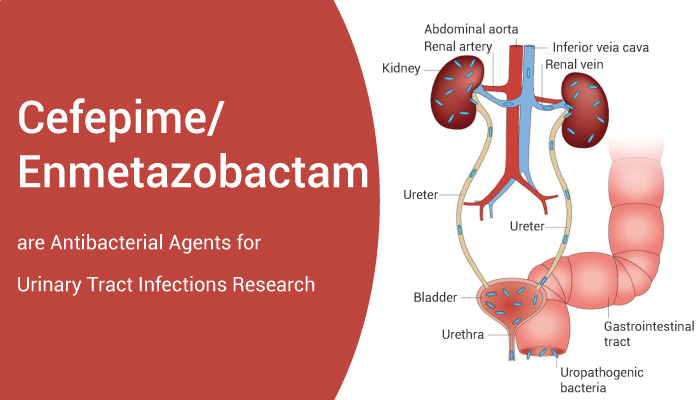
Urinary tract infections (UTIs) are common infections caused predominantly by uropathogenic Escherichia coli (UPEC). The annual incidence of physician-diagnosed UTIs in the United States is greater than 10% for females and 3% for males. Individual risk for infection depends on various factors, including age, sexual activity, family history, medical comorbidities and an individual history of UTI. Importantly, multi drug resistance (MDR) has spread rapidly through E. coli populations, making infections more troublesome and costlier to treat.
On February 22, 2024,FDA approves Cefepime/Enmetazobactam for cUTI treatment.
EXBLIFEP is a combination of Cefepime, a cephalosporin antibacterial, and Enmetazobactam, a beta-lactamase inhibitor.It indicated for the treatment of patients 18 years and older with complicated urinary tract infections (cUTI) including pyelonephritis caused by designated susceptible microorganisms.
In early research, Cefepime (BMY-28142) is a broad-spectrum and cross the blood-brain barrier cephalosporin. It shows antibacterial effects against both Gram-positive and Gram-negative aerobic bacteria. Furthermore, it induces neurotoxicity. In addition, enmetazobactam (AAI101) can against many resistant Gram-negative pathogens.
Belley et al. conducted a phase 3 interventional, explanatory, double-blinded randomised non-inferiority clinical trial involving 1034 randomised patients over 19 countries. Firstly, they compared the efficacy and safety of enmetazobactam and cefepime with tazobactam and piperacillin. Cefepime-enmetazobactam demonstrated a 79.1% efficacy at time of cure compared to 58.9% in piperacillin-tazobactam. Meanwhile, of the patients tested, 20.9% had ESBL producing uropathogens, 99.3% of which expressed a CTX-M type. Additionally, 3.5% co-expressed AmpC. Theseexhibit Cefepime-enmetazobactam presents another promising antibiotic.
In a word, FDA approved Cefepime/Enmetazobactam to treat complicated urinary tract infections.
Reference:
[1] Klein RD, et al. Nat Rev Microbiol. 2020 Apr;18(4):211-226.
[2] Walker MM, et al. Antibiotics (Basel). 2022 Dec 15;11(12):1821.