Recently, a team from West China Hospital of Sichuan University published a research paper. In this article, the team found a new immune checkpoint between circulating tumor cells (CTCs) and NK cells, HLAE.
First, researchers collect blood samples from the primary tumor, liver metastases and the hepatic portal vein (HPV) of patients with pancreatic ductal adenocarcinoma. Second, researchers utilized single-cell sequencing (scRNA-seq) and other technologies to analyze the transcriptomic features and gene expression differences. The results demonstrated a major interaction between CTCs and NK cells in the blood circulation. Third, HLA-E and CD94-NKG2 showed the strongest immune interaction between CTCS and NK cells. Researchers examined whether CTCs escaped from NK cell surveillance through the HLA-E: CD94-NKG2A checkpoint using an in vitro NK cytotoxicity assay. In vitro and in vivo analyses revealed that CTC and NK cells interacted with HLA-E: CD94-NKG2A via immune checkpoint molecules. Disruption of this interaction through NKG2A blocking or knocking down HLA-E expression increased NK-mediated tumor-cell killing in vitro.
Mechanism: CTC up-regulates the immune checkpoint molecule HLA-E by platelet-derived RGS18
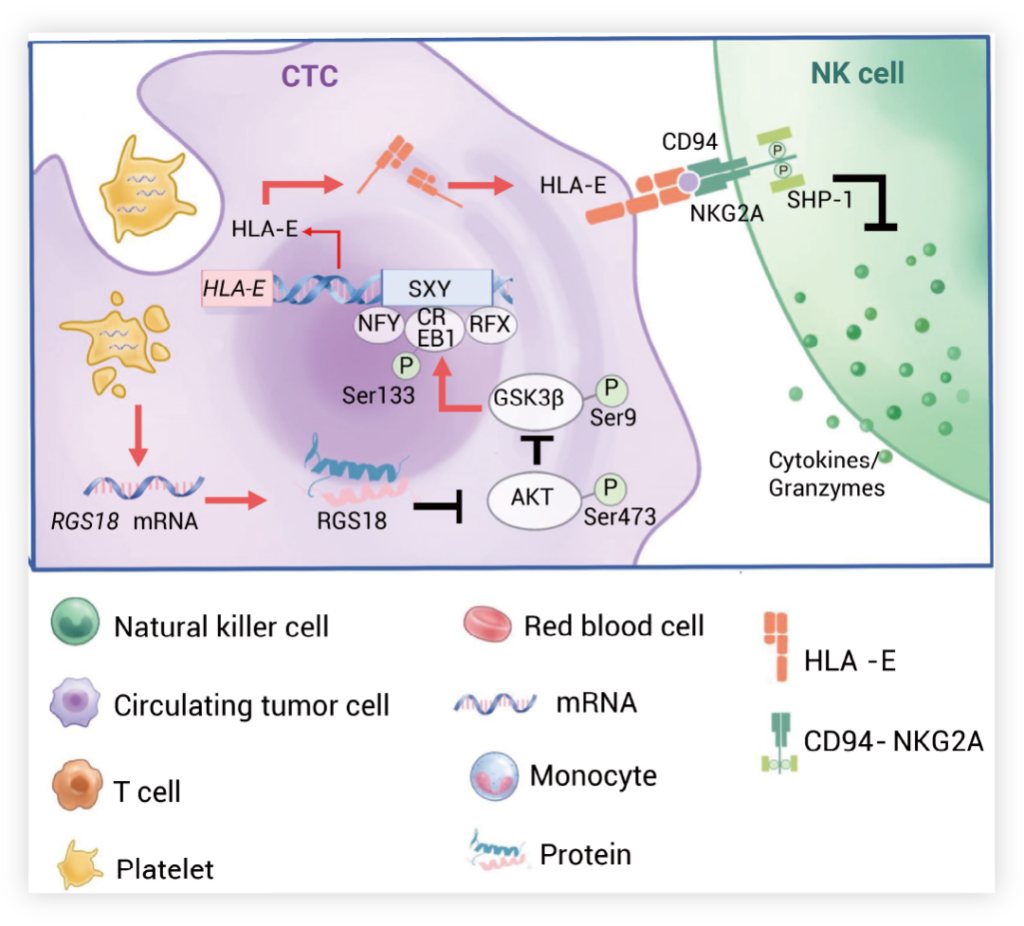
Once CTCs intravasate into blood vessels, they adhere to and absorb platelets carrying RGS18. RGS18 inhibits the activation of phosphorylation AKT in host cells, leading to the stabilization of the GSK3β protein through the suppression of GSK3β phosphorylation at Ser9. Furthermore, the GSK3β protein then promotes the nucleus translocation of CREB1 by phosphorylating CREB1 at Ser133. Inside the nucleus, CREB1 binds to the SXY site in the promoter region of the HLA-E gene, up-regulating HLA-E expression and its translocation to the cell surface of CTCs. Finally, the cell surface HLA-E interacts with CD94-NKG2A on NK cells, activating the intracellular phosphatase SHP1 and suppressing the cytotoxic activity of NK cells.
References:
[1] Korman AJ, et al. Adv Immunol. 2006;90:297-339.
[2] Liu X, et al. Cancer Cell. 2023;41(2):272-287.e9.
