Letermovir is a potent antiviral drug used for the treatment of cytomegalovirus (CMV) infections. It can be a serious complication in immunocompromised patients, particularly after organ transplantation. CMV infection can affect various organs and also weaken the immune system, leading to other infections and complications.
Letermovir is a potent inhibitor of CMV for Cytomegalovirus Infection Research
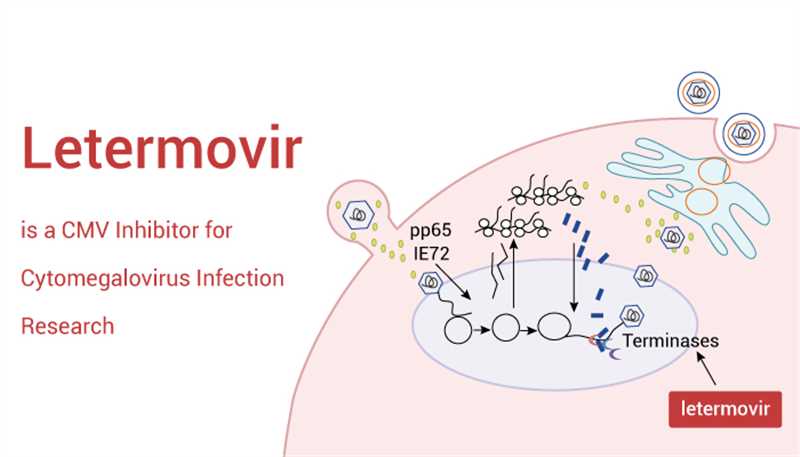
Letermovir (AIC246) is a potent inhibitor of CMV. It inhibits the terminal phase of the virus life cycle by targeting the subunit UL56 of the terminase enzyme complex. Here are some key points about Letermovir:
Mechanism of Action: Letermovir inhibits the CMV DNA terminase complex (UL56), which is essential for viral DNA processing and packaging. This unique mechanism of action makes it an attractive option for CMV prophylaxis or cases of multidrug-resistant CMV.
Efficacy: Letermovir has shown antiviral efficacy against human cytomegaloviruses, with remarkable selectivity. Meanwhile, it specifically targets the UL56 subunit of the terminase enzyme complex, inhibiting HCMV replication. In combination with other anti-HCMV drugs, Letermovir has additive antiviral effects.
Clinical Studies: Letermovir is currently being developed for the preventive treatment of CMV infections. In a phase II study, it demonstrated prophylactic efficacy in CMV-seropositive patients undergoing hematopoietic stem cell allograft. The study showed a reduced incidence of prophylaxis failure in patients receiving Letermovir compared to placebo.
Safety: Letermovir has uncommon side effects such as gastrointestinal symptoms and dyspnea. Side effects are comparable to those under placebo treatment.
In conclusion, Letermovir is a promising antiviral drug for the treatment and prevention of CMV infections, particularly in immunocompromised patients. So, Its unique mechanism of action and efficacy make it a valuable addition to the available treatment options.
Reference:
[1] P. Frange, et al. Médecine et Maladies InfectieusesVolume 48, Issue 8, December 2018, Pages 495-502.
[2] Marschall M, et al. Antimicrob Agents Chemother. 2012 Feb;56(2):1135-7.