On March 19, 2024, the U.S. Food and Drug Administration recently granted approval to Aprocitentan. Aprocitentan is a new medication aimed at addressing refractory hypertension in adults. Aprocitentan, a dual ETA/ETB antagonist, has shown promising results in both in vitro and in vivo studies.
Endothelin receptors, a type of G protein-coupled receptors, play a crucial role in regulating blood pressure. These receptors bind to endothelin ligands, which are peptides derived from longer prepro-endothelin precursors. There are four known types of endothelin receptors: ETA, ETB (ETB1, ETB2), and ETC. While the ETA receptor exhibits high affinity for ET-1 and ET-2 compared to ET-3, the ETB receptor displays equivalent high affinity for all three endothelin isopeptides.
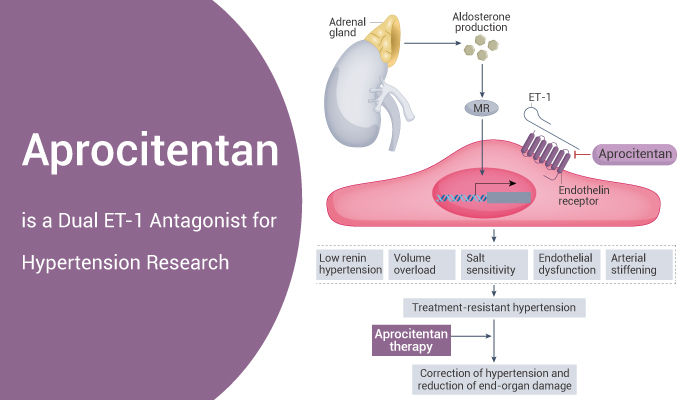
Aprocitentan: A Dual ET-1 Antagonist for in Hypertension Treatment
Aprocitentan, the primary active metabolite of Macitentan, acts as a potent antagonist for both ETA and ETB receptors. And it is dual ETA/ETB antagonist with IC50s of 3.4 nM and 987 nM, respectively. In vitro, Aprocitentan effectively inhibits intracellular calcium increase induced by ET-1 in various cell types, including primary human pulmonary smooth muscle cells, rat aortic smooth muscle cell line A10, and mouse fibroblast cell line 3T3.
Furthermore, Aprocitentan exhibits favorable pharmacokinetic properties in animal models. In Vivo, Aprocitentan has a volume of distribution greater than the plasma volume and a longer half-life than its parent compound in rat. The mean recovery of Aprocitentan in rat plasma ranges from 82.6% to 90.6%, indicating its efficient utilization in the body.
In conclusion, the approval of Aprocitentan marks an important milestone in the management of refractory hypertension. As further clinical studies ensue, Aprocitentan holds the potential to improve the quality of life for individuals struggling with resistant hypertension.
Reference:
[1]. J Pharmacol Exp Ther. 2008 Dec;327(3):736-45.
[2]. Int J Clin Exp Med. 2015 Oct 15;8(10):18420-6