Neutrophils are recruited into inflammatory areas to eliminate invasive pathogens in inflammatory process. Unfortunately, excessive recruitment and activation of neutrophils are harmful to human health. Moreover, that situation is involved in the progression of acute and chronic inflammatory diseases, including acute lung injury (ALI), rheumatoid arthritis, and sepsis. Especially, superoxide anion and photolytic enzymes released from activated neutrophils may facilitate tissue injury. In lung, the recruitment of activated neutrophils could cause endothelial damage. Formyl peptide receptor 1 (FPR1) is an emerging therapeutic target for the discovery of drugs to treat neutrophilic inflammatory diseases. However, development of FPR1 antagonists for clinical use is still inadequate. A study from Shun-Chin Yang discovered and identified HCH6-1 as a FPR1 antagonist.
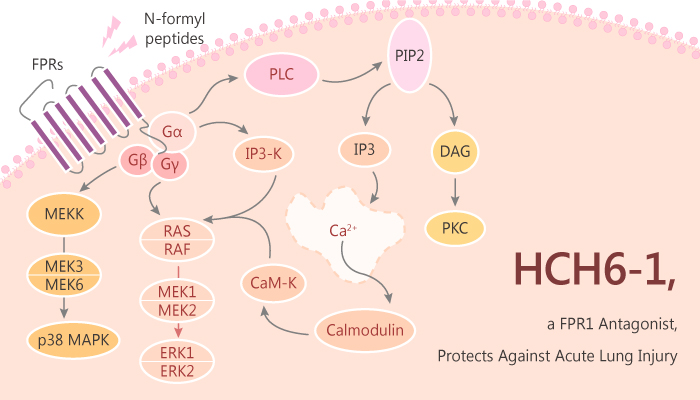
HCH6-1 is a competitive antagonist of Formyl peptide receptor 1 (FPR1). It is a FPR1 inhibitor and has protective effects against acute lung injury (ALI). HCH6-1 inhibits superoxide anion generation, elastase release, and chemotaxis in human neutrophils activated by fMLF (an FPR1 agonist). Besides, HCH6-1 did not attenuate inflammatory responses in non-FPR agonist-stimulated neutrophils, which suggested HCH6-1 as a selective FPR1 antagonist.
In the study, the authors found that HCH6-1 bound to FPR1, specifically inhibited chemotaxis, superoxide anion generation, and elastase release. In addition, HCH6-1 exhibited protective effects in LPS-induced ALI in mice. Moreover, HCH6-1 attenuated phosphorylation of mitogen-activated protein kinases (MAPKs) and Akt protein in fMLF-activated human neutrophils.
However, the exact pharmacological mechanism of HCH6-1 remains elusive, and its therapeutic potential in vitro and in vivo needs to be further explored.