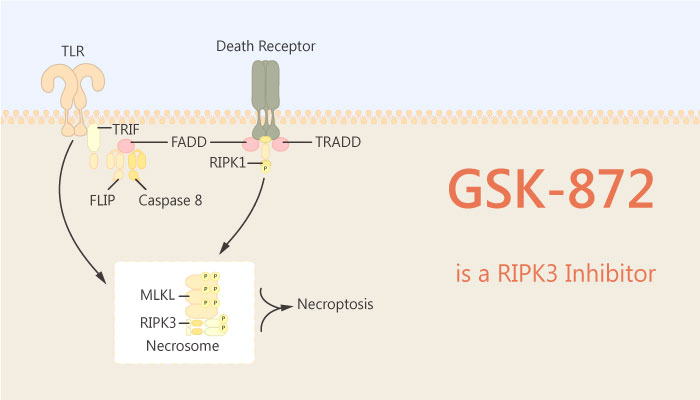
Necroptosis is a programmed form of necrosis, or inflammatory cell death. Especially, necroptosis is an alternative mode of regulated cell death mimicking features of apoptosis and necrosis. Necroptosis is triggered when apoptosis is inhibited. This death pathway contributes to host defense against infection as well as to inflammation. Apoptosis is characterized by a requirement for caspases. Crucially, necroptosis requires protein RIPK3 and its substrate mixed lineage kinase domain-like (MLKL), the crucial players of this pathway. Necroptosis depends on RIP3 kinase activation.
Receptor-interacting protein kinase 3 (RIPK3) is a serine/threonine protein kinase that mediates necroptosis. RIPK3 is a regulator of inflammation, cell survival, and disease. Besides, RIPK3 has emerged as a central player in necroptosis and a potential target to control inflammatory disease. RIP3 kinase activity supports the recruitment of the MLKL to trigger membrane leakage with the consequent release of proinflammatory intracellular components. Upon induction of necroptosis, RIPK3 phosphorylates its downstream effector mixed lineage kinase domain-like (MLKL). As a result, MLKL promotes oligomerization of MLKL on the plasma membrane, where it forms membrane pores to execute lytic cell death.
In this study, Pratyusha Mandal, et al identified RIP3 inhibitor GSK-872. GSK-872 binds RIP3 kinase domain with high affinity (IC50=1.8 nM). Moreover, GSK-872 inhibits RIP3 kinase activity (IC50=1.3 nM). All in all, GSK-872 is a potent, selective, and reversible RIPK3 inhibitor for blocking programmed necrosis.