The serine hydrolases monoacylglycerol lipase (MAGL) is responsible for degrading 2-arachidonoyl glycerol (2-AG). And the fatty acid amide hydrolase (FAAH) degrades N-arachidonoyl ethanolamine (anandamide or AEA). They are endogenous agonists for the cannabinoid receptors, CB1 and CB2. Meanwhile, The endocannabinoid system modulates a diverse set of physiological processes, including pain, inflammation, sleep, and appetite. So drugs that augment cannabinoid signaling may have therapeutic value. Thus, endocannabinoid hydrolase inhibitors have the potential to reduce pain and (neuro) inflammatory responses, improve neuropsychiatric function. And it is without substantial motor or cognitive impairment. Furthermore, In the nervous system and select peripheral tissues, MAGL regulates 2-AG, also arachidonic acid (AA) and AA-derived eicosanoids. Developing selective and in-vivo-active MAGL inhibitors as probes for studying MAGL function is important. MAGL inhibition produces opiate-sparing events with diminished tolerance, constipation, and cannabimimetic side effects. MJN110 is an orally active and selective monoacylglycerol lipase (MAGL) inhibitor.
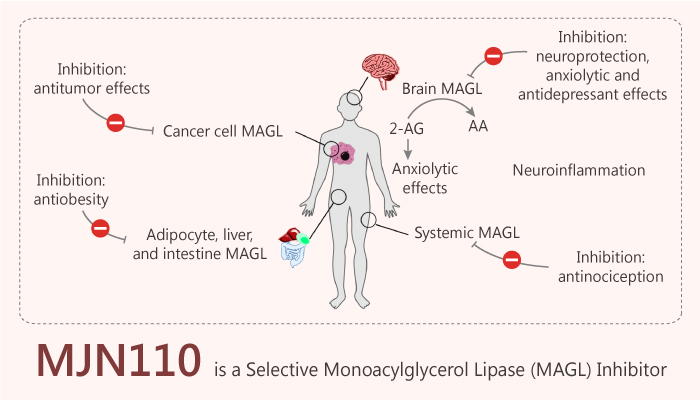
MJN110 is an orally active and selective MAGL inhibitor with IC50s of 9.1 nM and 2.1 nM for hMAGL and 2-AG, respectively. Besides, MJN110 produces opioid-sparing effects and displays strong antihyperalgesic activity. And, MJN110 also inhibited MAGL in vivo when administered intraperitoneally, with maximal inhibition observed at 1.0 mg/kg in the brain and 0.25 mg/kg in the liver. In addition, MJN110 reverses established mechanical allodynia in a rat model of diabetic neuropathy. Specifically, MJN110 reverses chronic constriction injury (CCI) -induced mechanical allodynia and thermal hyperalgesia in a dose-dependent manner. The respective ED50 value (95% confidence limits) is 0.430 (0.233-0.793) mg/kg. MJN110 has the primary serine hydrolase target, hMAGL, with an IC50 of ~1 nM. All in all, MJN110 is an orally active, potent and selective MAGL inhibitor.
References:
Niphakis MJ, et al. ACS Chem Neurosci. 2013 Sep 18;4(9):1322-32.