Toll-like receptors (TLRs) are type I transmembrane proteins. They can recognize pathogen-derived macromolecules, thereby playing a key role in the innate immune system. TLR dimerization leads to the activation of nuclear factor-κB (NF-κB) and interferon-regulatory factors (IRFs). These transcription factors, in turn, induce the production of proinflammatory cytokines and type I interferons (IFNs), respectively. TLR2, signaling as a heterodimer with either TLR1 or TLR6, recognizes a wide range of ligands. All in all, TLR1/2 is an important regulator of innate immunity. It provides an attractive target for the treatment of various immune disorders. In this study, CU-CPT22 is a potent protein complex of TLR1/2 inhibitor and competes with the synthetic triacylated lipoprotein (Pam3CSK4) binding to TLR1/2.
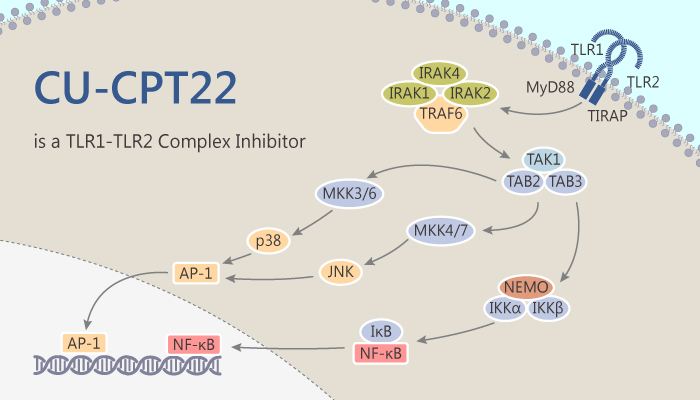
CU-CPT22 is a potent protein complex of TLR1/2 inhibitor and competes with Pam3CSK4 binding to TLR1/2.
CU-CPT22 possesses inhibitory activity for TLR1/2. It shows dose-dependent inhibitory effects blocking Pam3CSK4-induced TLR1/2 activation with an IC50 of 0.58 µM. Furthermore, CU-CPT22 competes with Pam3CSK4 for binding to TLR1/2 with a Ki of 0.41 µM. This is consistent with its potency observed in the whole-cell assay. Thus, CU-CPT22 can compete with Pam3CSK4 binding to TLR1/2. In addition, CU-CPT22 inhibits TLR1/2 signaling without affecting other TLRs, showing it is highly selective in intact cells. Moreover, CU-CPT22 has no significant cytotoxicity at various concentrations up to 100 µM in RAW 264.7 cells. it demonstrates minimal non-specific inhibition against a panel of 10 representative kinases (PDGFRB, MET, DDR2, SRC, MAPK1, PAK1, AKT1, PKC-γ, CAMK1, and PLK4). Lastly, CU-CPT22 can inhibit about 60% of TNF-α and 95% of IL-1β at 8 µM. Thus, CU-CPT22 suppresses TLR1/2-mediated inflammation response.
In summary, CU-CPT22 can compete with the synthetic triacylated lipoprotein (Pam3CSK4) binding to TLR1/2 with potency and specificity. It also suppresses TLR1/2-mediated inflammation response. So, it provides a much needed molecular probe for studying ligand interactions of the TLR1/2 protein complex.
Reference:
Cheng K, et al. Angew Chem Int Ed Engl. 2012 Dec 3;51(49):12246-9.