Phosphodiesterase (PDE) is any enzyme that breaks a phosphodiester bond. Usually, people speaking of PDE are referring to cyclic nucleotide PDEs. PDEs catalyse the hydrolysis of the phosphodiester bond of c-AMP and c-GMP affording the corresponding AMP and GMP inactive counterparts. In fact, the inhibition of PDE leads to an increase in cyclic nucleotide levels, which in turn play a prominent role as second messengers, in the regulation of a variety of cell functions, such as secretion, contraction, metabolism and growth. However, there are many other families of PDEs, including phospholipases C and D, autotaxin, sphingomyelin PDE, DNases, RNases, and restriction endonucleases, as well as numerous less-well-characterized small-molecule PDEs. The cyclic nucleotide PDEs comprise a group of enzymes that degrade the phosphodiester bond in the second messenger molecules cAMP and cGMP. They regulate the localization, duration, and amplitude of cyclic nucleotide signaling within subcellular domains. PDEs are therefore important regulators ofsignal transduction mediated by these second messenger molecules.
Since the early 1980s, phosphodiesterase 4 (PDE4) has been an attractive target for the treatment of inflammation-based diseases. These enzymes can mediate several physiological processes, such as brain functions, macrophage and monocyte activation, myocardial contractility, vascular smooth muscle proliferation and neutrophil infiltration, to name a few. Moreover, PDE4 participates in the physio-pathogenesis of many inflammatory diseases such as rheumatoid arthritis, chronic obstructive pulmonary disease (COPD) and asthma. Additionally, PDE4 have shown roles in the progress and development of autoimmune diseases, cardiovascular diseases, and cancers.
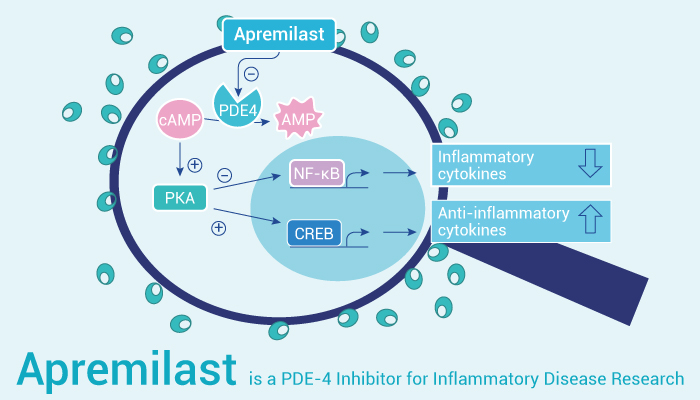
Apremilast is an orally active PDE4 inhibitor that can regulate inflammatory mediators.
Apremilast (CC-10004) is an orally available inhibitor of type-4 cyclic nucleotide PDE4. Importantly, Apremilast inhibits TNF-α release by LPS, which almost exactly replicates previous reported TNF-α inhibition by Apremilast on PBMCs and which is similar to the potency of Apremilast for PDE4 enzymatic inhibition. Meanwhile, PKA, Epac1 and Epac2 knockdowns prevented TNF-α inhibition and IL-10 stimulation by Apremilast. Apremilast significantly inhibits TNF-α production in the air pouch and diminishes the number of leukocytes present. In agreement, immunohistologic analysis shows that neutrophil accumulation in the air pouch membrane is dramatically reduced by Apremilast. In the murine air pouch model, both Apremilast and Methotrexate significantly inhibit leukocyte infiltration, while Apremilast, but not Methotrexate, significantly inhibits TNF-α release.
References:
[1] Crocetti L, et, al. Molecules. 2022 Aug 4;27(15):4964.
[2] Perez-Aso M, et, al. Arthritis Res Ther. 2015 Sep 15;17(1):249.