NOD2 (nucleotide-binding oligomerization domain 2) is an intracellular pattern recognition receptor. NOD2 is one of cytoplasmic PRRs, and antigen-presenting cells and certain mucosal epithelia primarily express it. It assembles with RIP-2 kinase in response to the presence of bacterial muramyl dipeptide (MDP) in the host cell cytoplasm. Thereby, it induces signals leading to the production of pro-inflammatory cytokines. Various inflammatory disorders show association with dysregulation of NOD2 signaling. Thus, NOD2 is a potential target for therapeutic intervention. In this study, GSK717 is a potent, selective NOD2 inhibitor. GSK717 inhibits muramyl dipeptide (MDP)-induced NOD2-mediated signaling. In addition, it has an IC50 of 400 nM for MDP-stimulated IL-8 secretion in HEK293/hNOD2 cells.
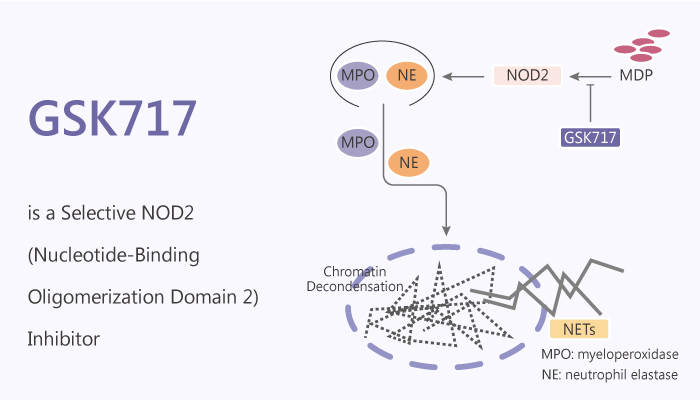
NOD2 may undergo a conformational change upon binding the bacterial cell wall component muramyl dipeptide (MDP) to enable ATP binding, oligomerization, and recruitment of the serine/threonine kinase RIP2. In turn, this leads to the recruitment of additional effector kinases including TAK1/TAB1 and the formation of a poly-ubiquitinated signaling complex that stimulates canonical NF-κB and MAPK (p38, JNK) pathways. Thereby, it leads to increased synthesis of pro-inflammatory cytokines and chemokines. In addition, NOD2 increases inflammatory cytokine production by macrophages and dendritic cells synergistically with several members of the toll-like receptor (TLR) family of membrane-associated PRRs. Fortunately, GSK717 also blocks synergy between NOD2 and TLR2. Moreover, GSK717 does not affect NOD1, TNFR1, and TLR2-mediated responses. Furthermore, GSK717 (5 μM) inhibits the release of IL-8, IL-6, TNFα, and IL-1β in primary human monocytes stimulated with MDP.
In summary, GSK717 is a potent, selective NOD2 inhibitor, and it has the potential to investigate the importance of NOD2 in various inflammatory processes and may have potential clinical utility.
Reference:
Rickard DJ, et al. PLoS One. 2013;8(8):e69619. Published 2013 Aug 1.