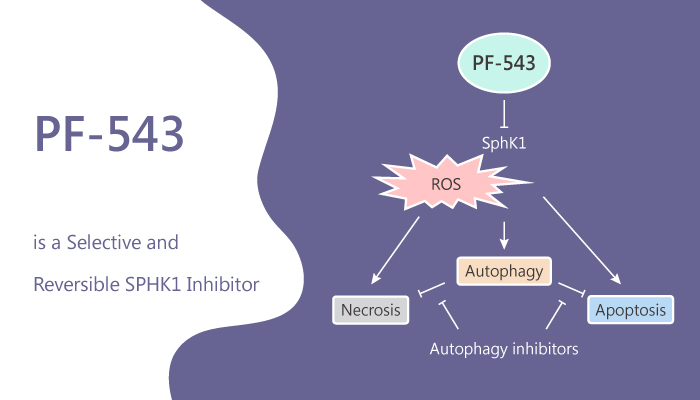
S1P (sphingosine 1-phosphate) is an extracellular ligand at five cognate GPCRs and a consequential intracellular lipid intermediate. It affects diverse cellular processes including migration, growth, and survival. S1P regulates the function and trafficking of immune cells, induces angiogenesis, and regulates endothelial cell functions. It also promotes the survival and migration of cancer cells. S1P is formed primarily in the intracellular compartments by the action of SphKs. In mammalian organisms, there are two isoforms of SphK1 and SphK2. Many types of tumors and cancer cells overexpressed SphK1. In this study, PF-543 is a potent, selective, reversible, and sphingosine-competitive SPHK1 inhibitor with an IC50 of 2 nM and a Ki of 3.6 nM. PF-543 is >100-fold selectivity for SPHK1 over SPHK2. PF-543 is also an effective potent inhibitor of S1P formation in whole blood with an IC50 of 26.7 nM. In addition, it also induces apoptosis, necrosis, and autophagy.
PF-543 is a potent, selective, reversible, and sphingosine-competitive SPHK1 inhibitor.
PF-543 inhibits C17-S1P formation in 1483 cells with an IC50 of 1.0 nM. Moreover, SphK1 inhibition by PF-543 causes a dose-dependent depletion of the intracellular level of S1P with an EC50 concentration of 8.4 nM. It also displays a concomitant elevation of the intracellular level of sphingosine in 1483 cells. Moreover, the level of endogenous S1P in 1483 cells after a 1 h treatment with 200 nM PF-543 is decreased 10-fold, producing a proportional increase in the level of sphingosine. In addition, PF-543 abolishes SK1 expression at nM concentrations. Furthermore, PF-543 also induces caspase-3/7 activity. It has no effect on vascular remodeling but reduces right ventricular hypertrophy. The protection involves a reduction in the expression of p53 and an increase in the expression of anti-oxidant nuclear factor Nrf-2.
In summary, PF-543, a potent inhibitor of SphK1, will be useful for dissecting specific roles of SphK1-driven S1P signaling.
Reference:
Schnute ME, et al. Biochem J. 2012 May 15;444(1):79-88.;MacRitchie N, et al. Cell Signal. 2016 Aug;28(8):946-55.