Histone deacetylases (HDAC) are a class of enzymes that remove acetyl groups from an ε-N-acetyl-lysine amino acid on a histone. Specifically, selective HDAC inhibitors are as a potential anti latent therapy for the treatment of persistent human immunodeficiency virus type 1 (HIV-1) infection. Besides, HDAC3 significantly induced expression from the HIV-1 promoter in the 2D10 latency cell line model. However, HDAC1 or HDAC2 alone or in combination did not significantly induce HIV-1 expression. Co depletion of HDAC2 and HDAC3 resulted in a significant increase in HIV-1 promoter expression. In addition, HDAC1, HDAC2 and HDAC3 knockout significantly increased HIV-1 promoter expression. HDAC3 enzymatic inhibition is crucial for induction of expression from quiescent HIV-1 proviruses. Moreover, selective HDAC inhibitors might have fewer effects on other cellular genes and less clinical toxicity. BRD3308 is a highly selective HDAC3 inhibitor and activates HIV-1 transcription.
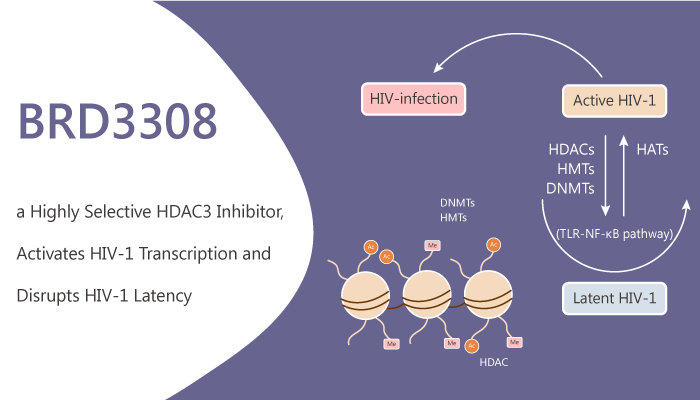
But, how does BRD3308 work on the target? Let’s discuss it in detail. BRD3308 is 23-fold selectivity for HDAC3 (IC50 of 54 nM) over HDAC1 (IC50 of 1.26 μM) or HDAC2 (IC50 of 1.34 μM). In addition, BRD3308 suppresses pancreatic β-cell apoptosis induced by inflammatory cytokines or glucolipotoxic stress. Meanwhile, BRD3308 increases functional insulin release. BRD3308 activates HIV-1 transcription and disrupts HIV-1 latency. Nonetheless, BRD3308 is able to induce outgrowth of HIV-1 from latently infected cells ex vivo in resting CD4+ T cells. Furthermore, BRD3308 treatment reduces hyperglycemia and increases insulin secretion in a rat model of type 2 diabetes. BRD3308 preserves the functional β-cell mass against glucolipotoxicity in vivo. All in all, BRD3308 is a highly selective HDAC3 inhibitor and activates HIV-1 transcription.
References:
Barton KM, et al. PLoS One. 2014 Aug 19;9(8):e102684.